
If a special infection occurs that cannot be killed or removed using normal sterilization methods, we will identify the set or equipment suspected of being infected based on the usage history and sterilization history of the equipment
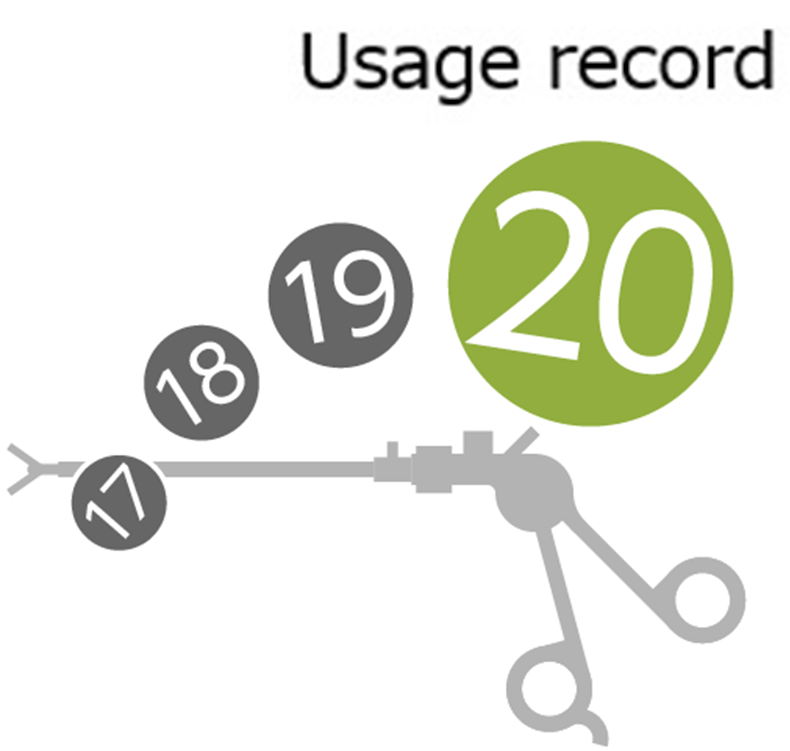
Preventing accidents caused by exceeding the durability limit of rigid endoscopic forceps, etc For example, excessive use of instruments such as rigid endoscopic forceps that have a set number of uses can lead to medical accidents. ''Chuzai Meijin'' records the number of times surgical equipment is used(1)and prepares for damage accidents. Additionally, these data(2)can be used for repair and purchasing planning

Preparing surgical equipment must be done quickly and accurately. ''Chuzai Meijin'' clearly expresses the required number of equipment and the actual number of equipment in stock, preventing omissions and mistakes in stocking

'Chuzai Meijin" records the sterilization history(3)and warns you when the sterilization period has expired. It also alerts you to unsterilized items and differences in sterilization methods
EBA Co., Ltd.OR&CSS Solution
+81 52-891-1208
Mon-Fri 9:00~17:00(Excluding Saturdays, Sundays, holidays, and company holidays)